VALIDATION
Definition and Goal of Validation
Validation is the process of establishing documentary evidence demonstrating that a process,
method , Equipment/Facility or system meet the requirement and quality factor.
Most of the drugs are irreplaceable in the event of side effect.
(It is impossible to check whether there is any defect due to the collection of samples from the final product,
and this would cost one's life in the event of side effects)
Validation: Development History
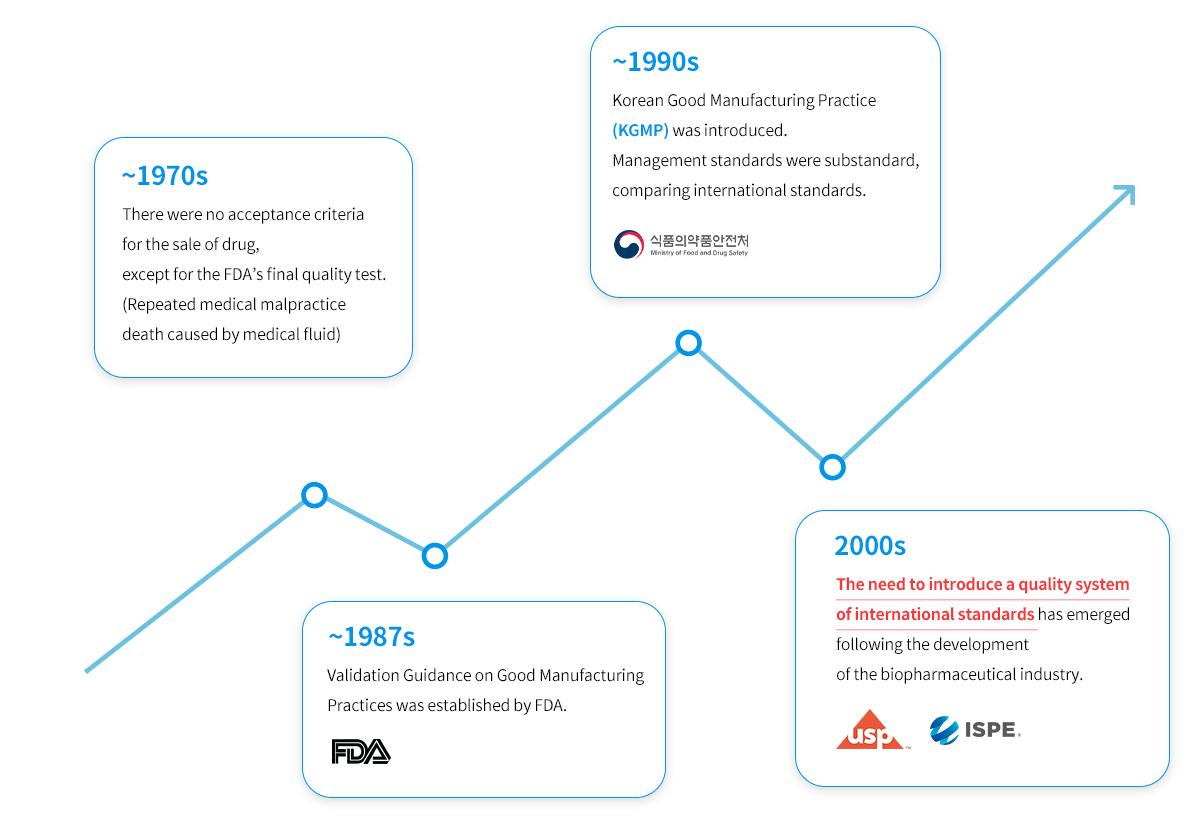
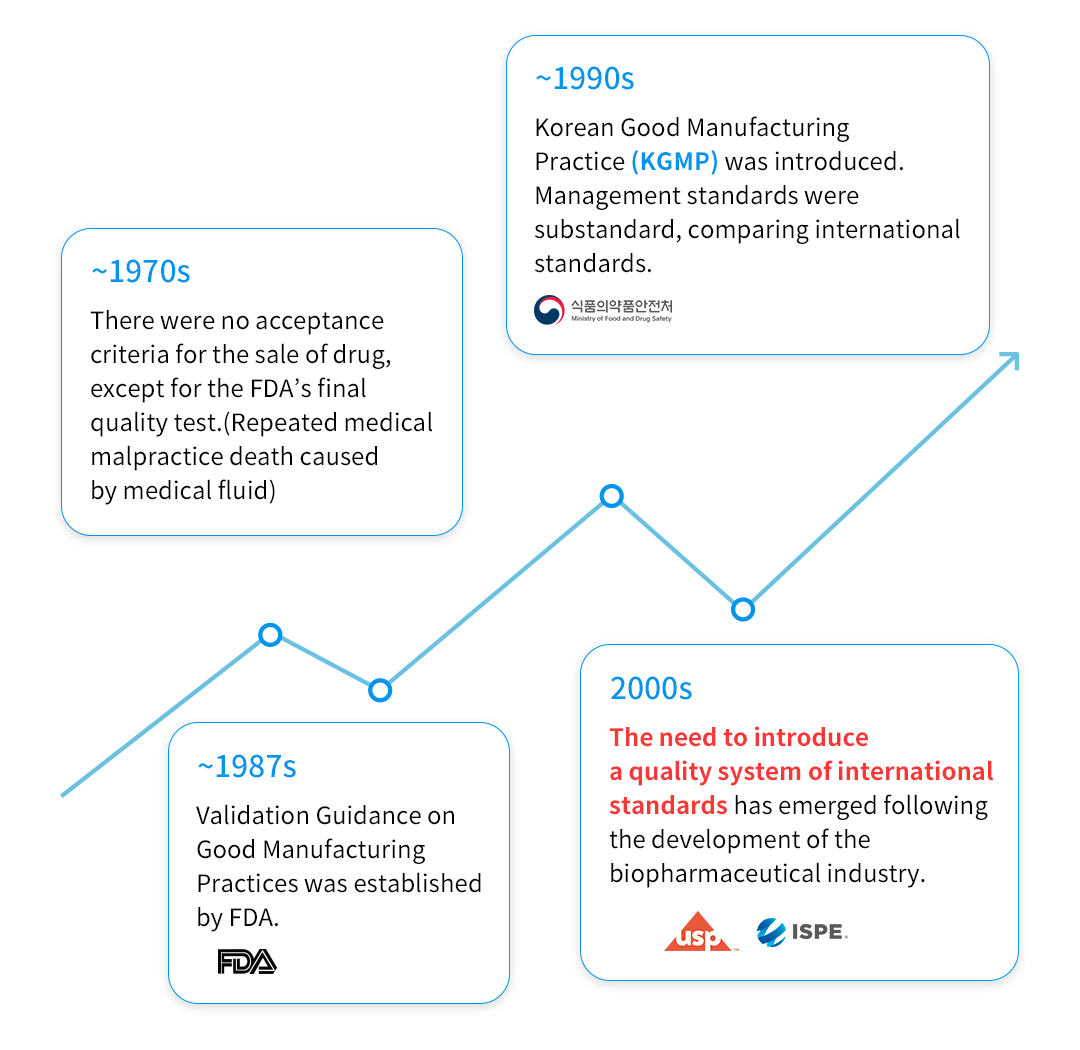
Systematization and Standardization of Material Prepared during Project Execution
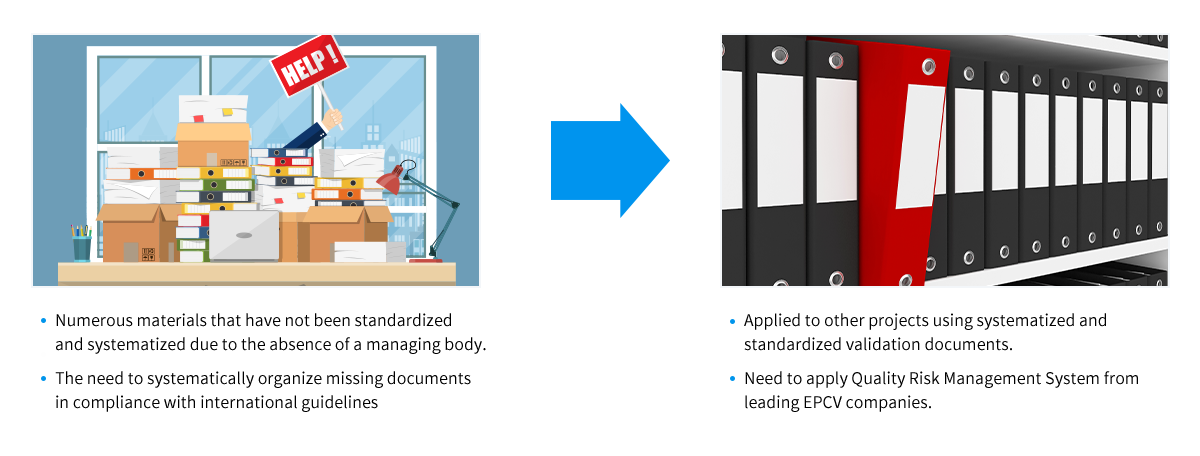
- ※ International Guideline for Reference
-
- ASTM E2500, Standard Guide for Specification, Design, and Verification of Phamaceutical and Biopharmaceutical
Manufacturing Systems and Equipment - ICH Q9 Quality Risk Management / ICH Q10 Pharmaceutical Quality System
- ISPE Baseline® Pharmaceutical Engineering Guides for New and Renovated Facilities, Volume 5: Commissioning and Qualification
- PIC/S Validation Master Plan, Installation and Operational Qualification, Non-sterile Process Validation, Cleaning Validation
- 7.1.11 GAMP 5 Guide: A Risk-Based Approach to Compliant GxP Computerized Systems
- ASTM E2500, Standard Guide for Specification, Design, and Verification of Phamaceutical and Biopharmaceutical
International Guideline Trend [Quality by Design]
- A systematic approach that emphasizes understanding and process management of products and processes based on Scientific Approach and Quality Risk Management
- Validation preparation through Good Engineering Practice (GEP) application from the beginning of the design of Facility

Data Integrity
- Maintaining and ensuring the accuracy and consistency of data throughout its life cycle against bio/pharmaceutical equipment or systems to which a computer system applies.
- Attributable, Legible, Contemporaneous, Original, Accurate.
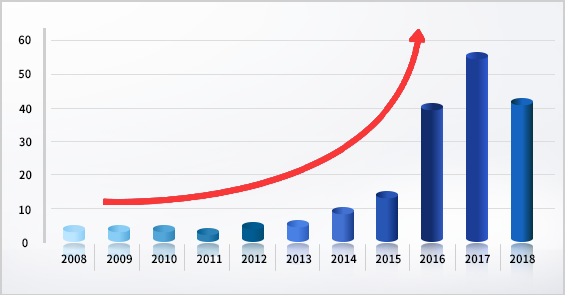
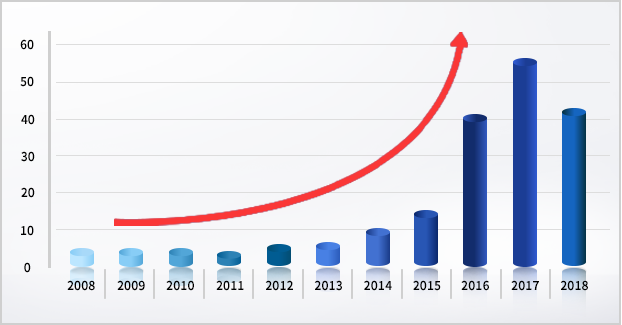
ALCOA Principle - Statistics since 2008 show that the number of Warning Letters related to data integrity has been on the rise for about 11 years.
- It has recently emerged as a major inspection point for Regulatory Authority's audits for pharmaceutical companies.
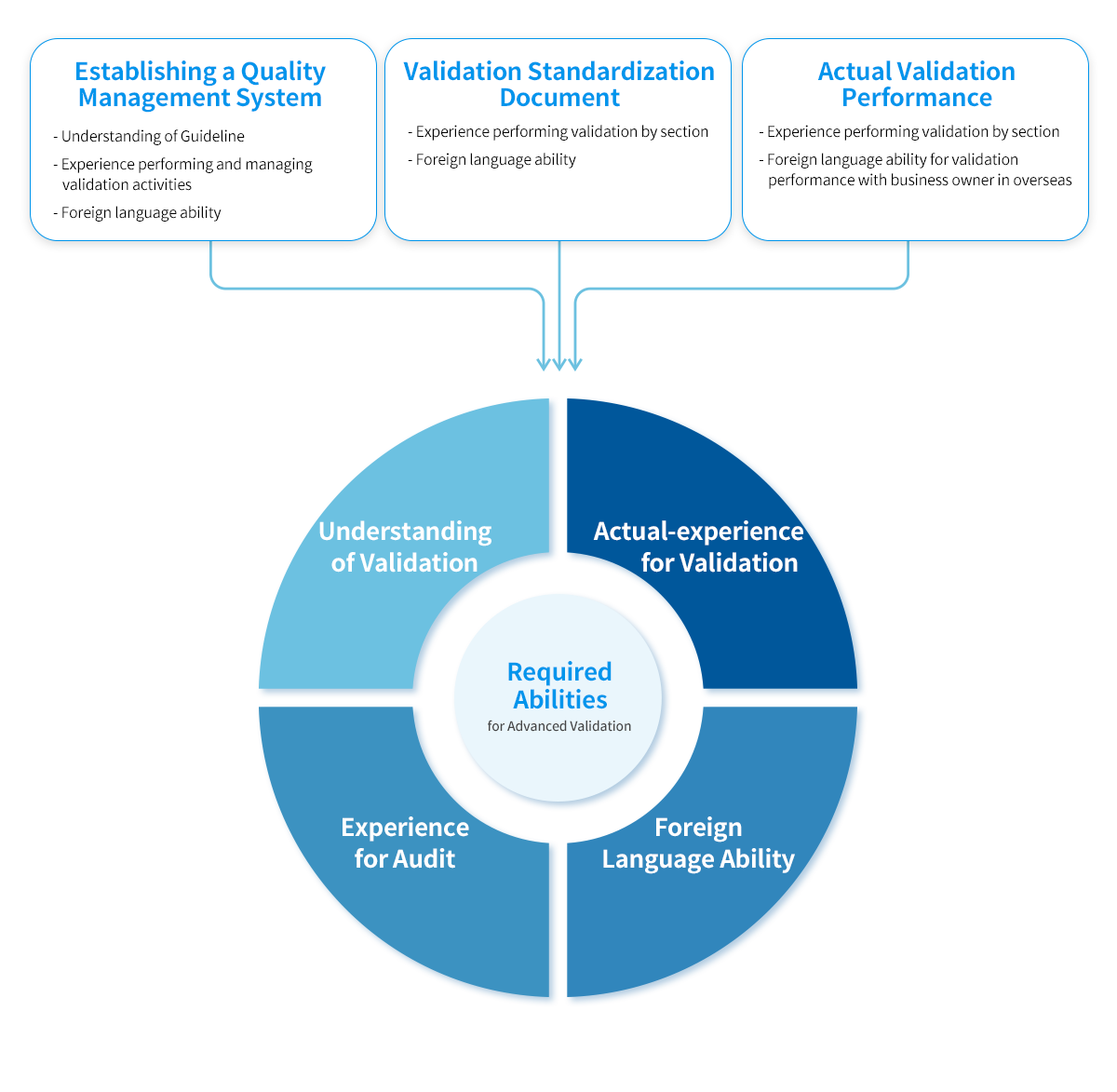
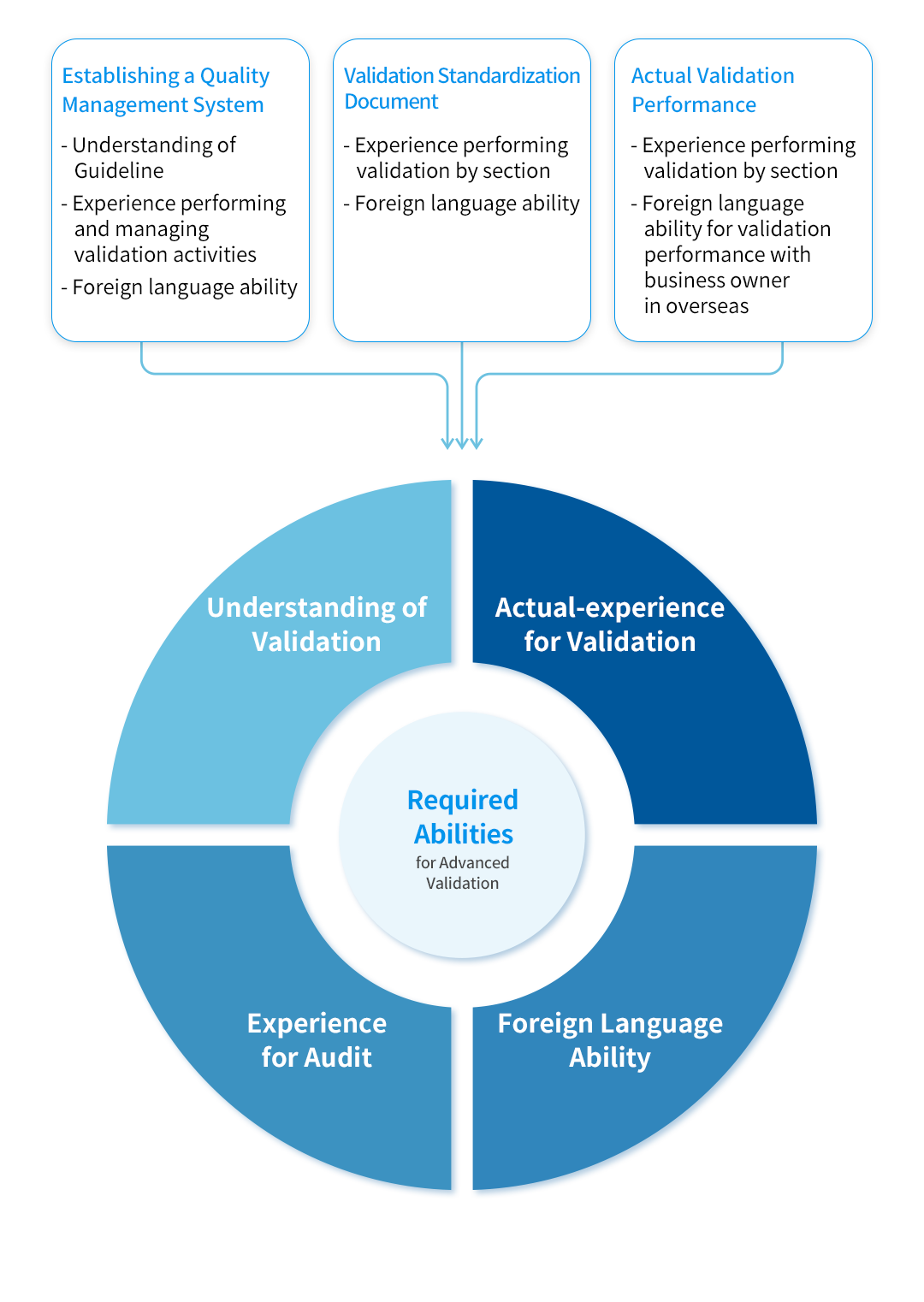
KBE Capabilities